
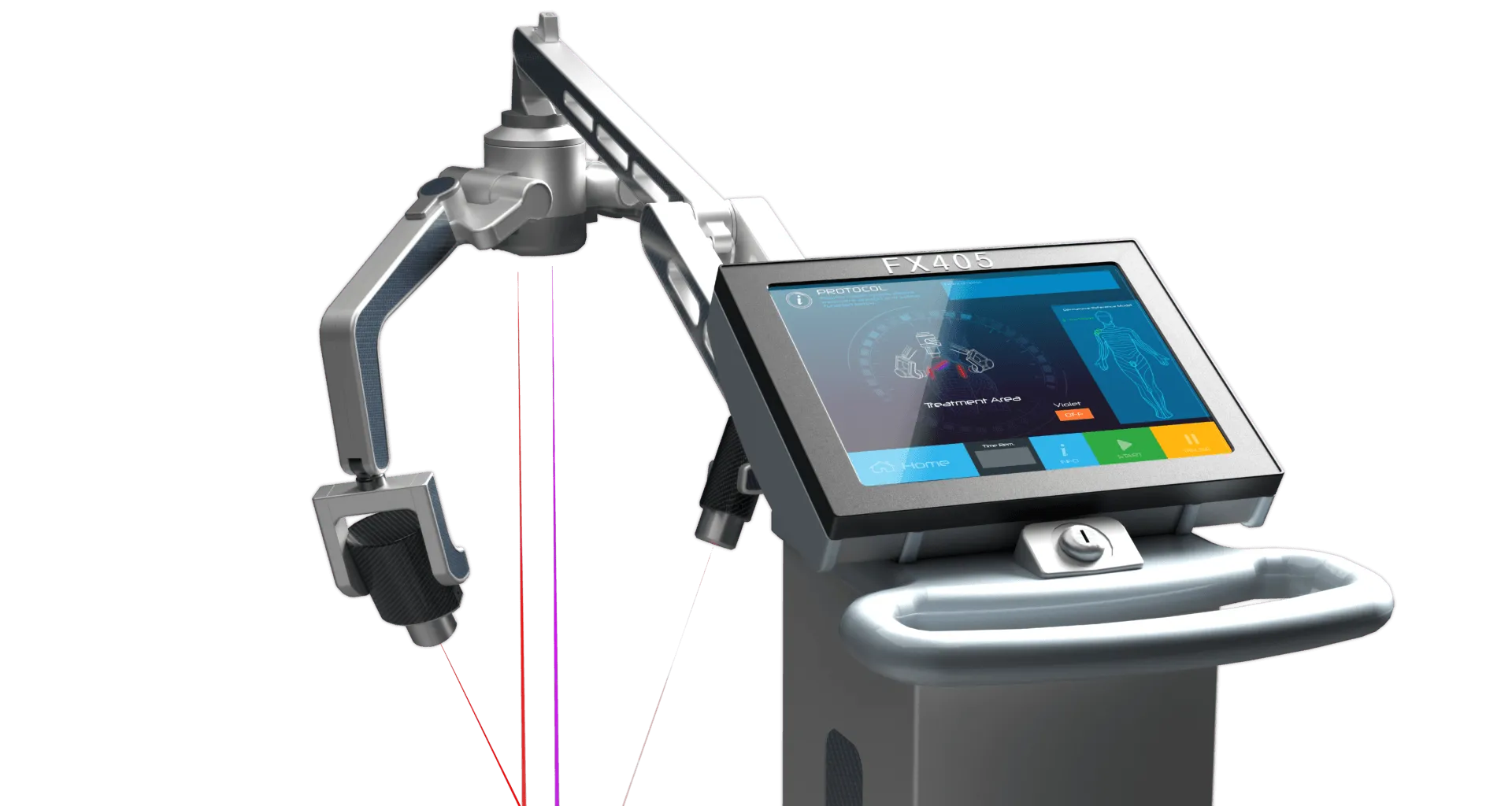
FX 405 Laser
The FX 405 laser scanner offers all of the same benefits that the FX 635 does, with the advantage of adding violet laser for versatility in treatments.
World Leads in Low Level Laser Technology
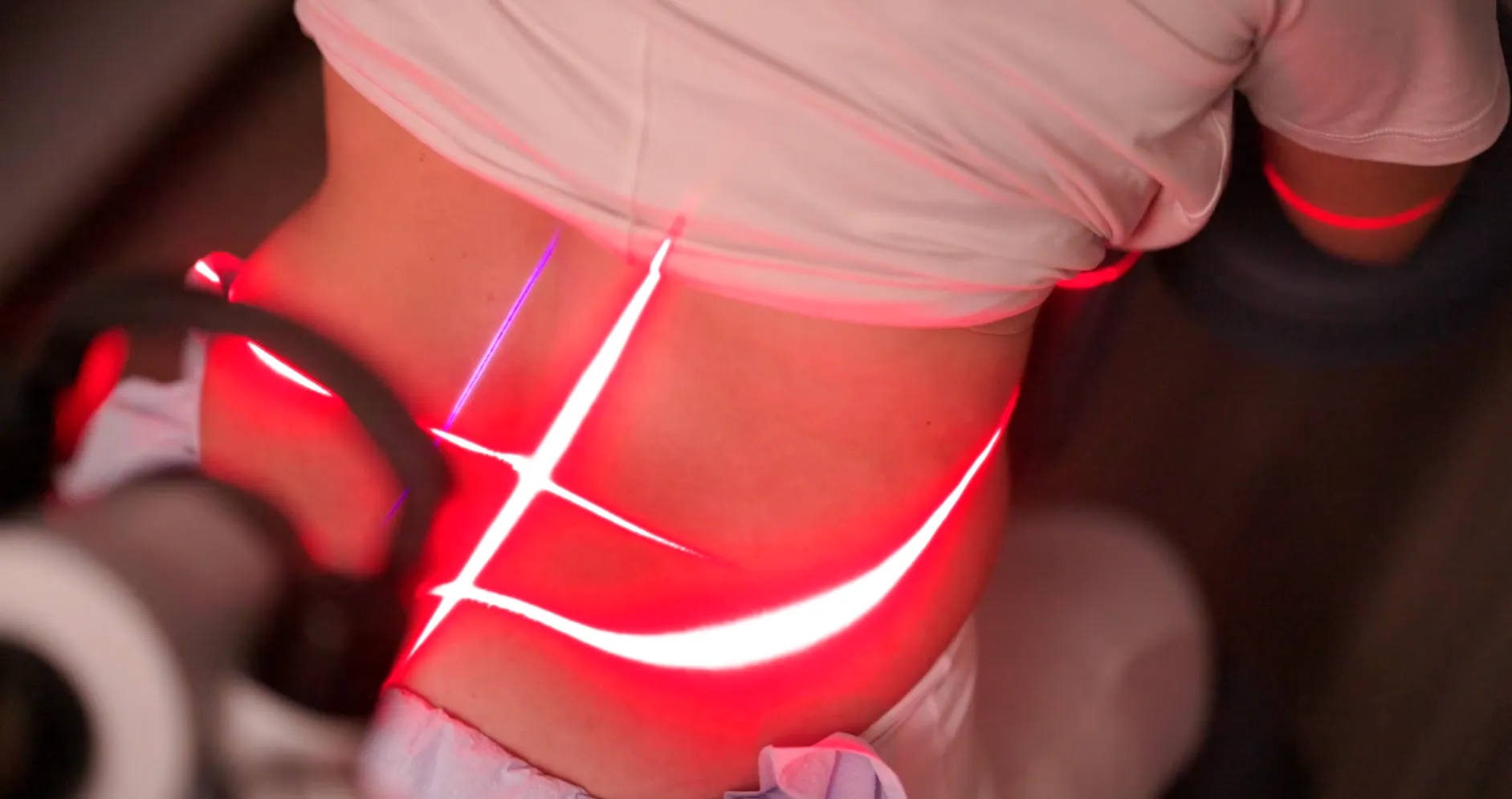
In addition to the violet laser’s anti-bacterial and anti-microbial properties, it also produces more energy per photon which research shows that the combination of violet and red lasers results in greater pain relief. The violet laser also enhances reactive oxygen species (ROS), which plays an important role in facilitating pain and inflammation reduction.
Product Description
The FX 405 uses low-level laser technology and patented laser diode arms to precisely target pain centers. The body absorbs the light energy, which encourages the cell’s mitochondria to produce more ATP and promotes natural healing within the cell.
Because the laser merely stimulates the cells to promote healing naturally, the FX 405 provides pain relief and reduces inflammation with no adverse side effects.

Treatment
Users remain awake throughout the entire laser treatment and experience no burning, scarring or downtime, allowing them to resume the rest of their day as soon as the session is over.
Specifications
- Configuration: (3) Class 2 17.25mW Line
- Generating Diodes
- Wavelength: 635nm & 405nm
- Modulation: Variable
- Weight: 65lbs (29.48 kg)
- Height: 70in (177.8 cm) (Average – Adjustable)
- Two Independent Adjustable Arms For Desired Laser Concentration
- 4 Locking Anti-Static Casters
- Display: Full Color Touch Screen Control
- Power Source: 100-240VAC 50-60Hz
- Chassis: Powder Coated Aircraft Aluminum
- Housing & Covers: Non-Allergenic Material, Spray Finished
- Accessories: 2-Keys, Power Cord, Laser Safety Glasses
- Compliant to: ISO 13485 Medical Device Quality, IEC 60825-1 Laser Safety, IEC
- 60601-1-2 EMC, IEC 60601-1 Safety, CE Mark, CB Certified
- FDA Laser Class 2 / FDA Device Class ll / EU Device Class IIa, Laser Class II
How it works
For Medical Professionals
Medical professionals have trusted Erchonia’s low-level lasers for treating various conditions for over 25 years and are increasingly turning to the valuable benefits of LLLT to manage a range of conditions more safely and effectively.
With the FX 405 medical staff can spend less time performing each treatment and use this valuable time to treat more patients and perform necessary tasks. Thanks to its triple-head, hands-free design, the medical professional just has to set up the FX 405 and go. This allows the physician to see other patients while the FX 405 does its work.
Are you ready to see just how easy and effective the low-level FX 405 laser is to implement into your practice?
FDA Erchonia Pain Market Clearances
Erchonia complies with the highest clinical testing. All Erchonia level (1) clinical trials have gone through the FDA pre-IDE process, IRB approval, and are based on pilot research to ensure customers that the laser they are purchasing has been proven safe and effective by the FDA.
90% of all medical devices are substantially equivalent with no clinical research, of the 10% that are, only few submit blinded and controlled studies. United States Food and Drug Administration

